From KERA:
A number of pharmaceutical companies have entered the home stretch in the race to develop a COVID-19 vaccine: human trials.
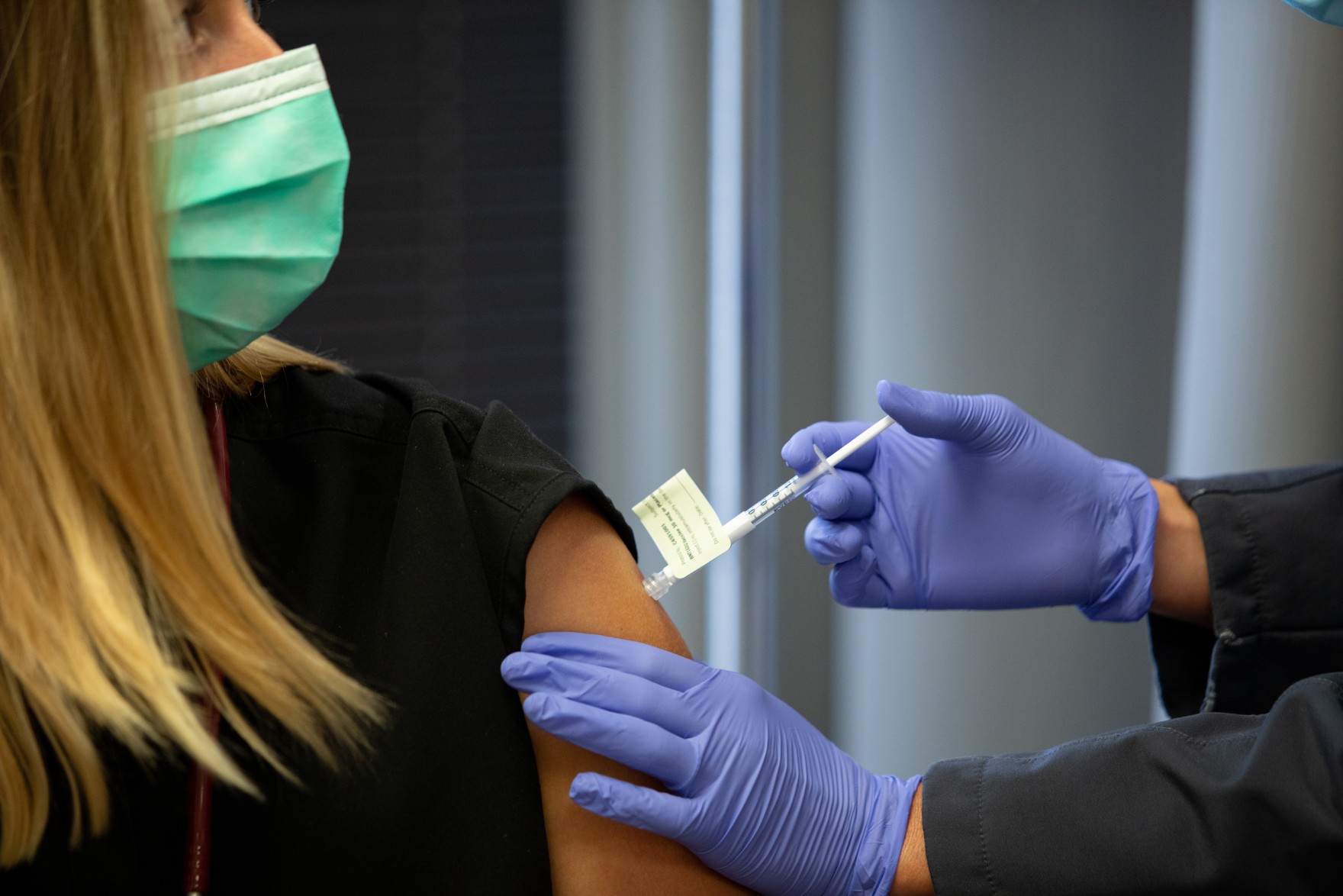
One of those trials is being conducted at Baylor University Medical Center in Dallas, and Ashley Agura is the perfect candidate. As a Physician Assistant, she spends 12 hours a day taking care of COVID-19 patients. A typical day for Agura starts around 7 a.m.
“We kind of glue your mask on for the day — your N-95, plus a droplet mask over that, eye shield — and then go visit with those patients and see how they’re doing,” she said. “Check in with their oxygen, check in with their symptoms.”

Dr. Mezgebe Behre talks with patient Ashley Agura at Baylor University Medical Center about the COVID-19 vaccine trial. It’s been two weeks since Agura took part in the trial, and she says she “feels great.”
Before Agura received her first injection of the experimental vaccine, she watched a video about how the process would work and the risks involved. She gave her informed consent and went through a few tests. Then, she received the first dose of either the vaccine or a placebo. About three weeks later, she got the second dose.
No one, not even the doctors, knows which she received. The first dose didn’t bother her much; it just left her with a sore arm. But after the second injection, she experienced mild side effects.
“I definitely had much more arm soreness and I actually felt a little bit, not ill the next day, but I definitely had body aches and a low grade temperature, which was not bothersome,” Agura said. “I worked the whole day and I just took some Advil.
That’s a good sign, said Dr. Mezgebe Berhe. He’s the lead researcher with the North Texas Infectious Disease Consultants, the team working on this trial.

Dr. Mezgebe Behre poses for a portrait in his office. / Keren Carrión
“You are actually trying to simulate a natural infection. So if you had COVID-19 virus, you’re expected to develop chills, pain, maybe fever,” Berhe said.
He said this trial vaccine is unique because it’s using the body’s own RNA. Messenger RNA, or mRNA, tell the body which proteins to build. Scientists have found a way to code mRNA to produce proteins that fight COVID-19.
“The whole concept is how do I expose the body to a specific protein of interest, so the body can mobilize a more targeted immune response,” he said.
This technology has been around for a few years, but it hasn’t been used in a vaccine before.
Pfizer is sponsoring this vaccine trial — Baylor is just one of the medical centers involved. Pfizer’s CEO Albert Bourla told CBS’s Face the Nationlast week that enough data will be collected by the end of October to be ready for FDA approval.
“But of course, that doesn’t mean that it works,” Bourla said. “It means we will know if it works.”

Sheila Smith, a pharmacy technician, prepares the vaccine candidate at Baylor University Medical Center. / Keren Carrión
Substantial public skepticism could throw a wrench in things, though. About one in three Americans say they wouldn’t get a free COVID-19 vaccine approved by the FDA, according to a recent Gallup Poll.
Dr. Peter Hotez is the Dean of the National School of Tropical Medicine at Baylor College of Medicine in Houston. He said he understands people’s concerns, especially because of Operation Warp Speed. That’s the federal government’s effort to speed up the development of COVID-19 treatments and vaccines.
“So far, the communication out of Operation Warp Speed has been largely non-existent, and I’m not sure for something of this profile and magnitude and concern, whether that will be adequate,” Hotez said. “So there’s going to have to be a higher level of communication with the American people.”
Bourla said that’s part of the reason he declined federal funding for his company’s vaccine. He wants to keep politics out of it.
“Whenever you get money from someone, that always comes with strings. They want to see how you are going to progress, what type of moves you are going to do, they want reports,” Bourla said. “I didn’t want to have any of that. Basically I gave them an open checkbook, so they can worry about scientific challenges and not anything else.”

Sheila Smith, a pharmacy technician, prepares the vaccine at Baylor University Medical Center. / Keren Carrión
Agura, the trial participant, said she trusts the science behind the Pfizer vaccine, but she is concerned about the long-term effectiveness.
“It’s still too early to know, are you going to have antibodies going into next year?” she said. “Because that’s just not enough time to test that.”
In the meantime, Dr. Berhe — the lead researcher on the Baylor trial — says Pfizer wants to recruit 10,000 more volunteers for its human trials, specifically minorities.
“If the minority are not enrolled in a study, it’s very difficult to understand the efficacy of this intervention in that sector of the population,” Berhe said.
Even though FDA approval isn’t guaranteed, Pfizer’s already started manufacturing its vaccine so it can be ready to distribute it as soon as possible.
CDC director Dr. Robert Redfield recently told Congress that won’t be possible until next year, but Berhe’s optimistic it will happen before the end of the year, and he said he expects an accelerated FDA approval process. Berhe said that’s not unheard of if there’s overwhelming data that the vaccine is effective.
Got a tip? Email Rebekah Morr at rmorr@kera.org. You can follow her on Twitter @bekah_morr.